Descripción
Hay varias formas de marcar un oligonucleótido con fluoresceína. La elección de la etiqueta se diversifica aún más, dependiendo de los requisitos espectrales. La fosforamidita de 5′-fluoresceína-CE, derivada del isómero único 6-carboxifluoresceína, la fosforamidita de 5′-hexaclorofluoresceína-CE (HEX) y la fosforamidita de 5′-tetraclorofluoresceína-CE (TET) se pueden usar para marcar eficientemente un oligonucleótido en el 5 ‘-fin. El etiquetado del extremo 3′ de un oligo con fluoresceína se puede lograr utilizando CPG de 3’-fluoresceína. La escisión estándar y la desprotección con hidróxido de amonio liberan el oligo marcado con fluoresceína cuando se usa cualquiera de estos soportes.
| Catalogo | Producto | Presentación |
|---|---|---|
| AAT-6026 | 6-HEX phosphoramidite [5′-Hexachlorofluorescein phosphoramidite] | 100 µmoles |
| AAT-6024 | 6-HEX phosphoramidite [5′-Hexachlorofluorescein phosphoramidite] | 10×100 µmoles |
Importante, Solo para uso en investigación (RUO). Almacenamiento: Congelar (< -15 °C), Minimizar la exposición a la luz
Propiedades fisicas
| Peso Molecular | 1050.61 |
| Disolvente | MeCN |
Calculadora
Preparación de la solución de stock común.
Volumen de MeCN necesario para reconstituir la masa específica de fosforamidita de 6-HEX [fosforamidita de 5′-hexaclorofluoresceína] a la concentración dada. Tenga en cuenta que el volumen es solo para preparar la solución madre. Consulte el protocolo experimental de muestra para conocer los buffers experimentales/fisiológicos apropiados.
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 95.183 µL | 475.914 µL | 951.828 µL | 4.759 mL | 9.518 mL |
| 5 mM | 19.037 µL | 95.183 µL | 190.366 µL | 951.828 µL | 1.904 mL |
| 10 mM | 9.518 µL | 47.591 µL | 95.183 µL | 475.914 µL | 951.828 µL |
Espectro
Abrir en Advanced Spectrum Viewer
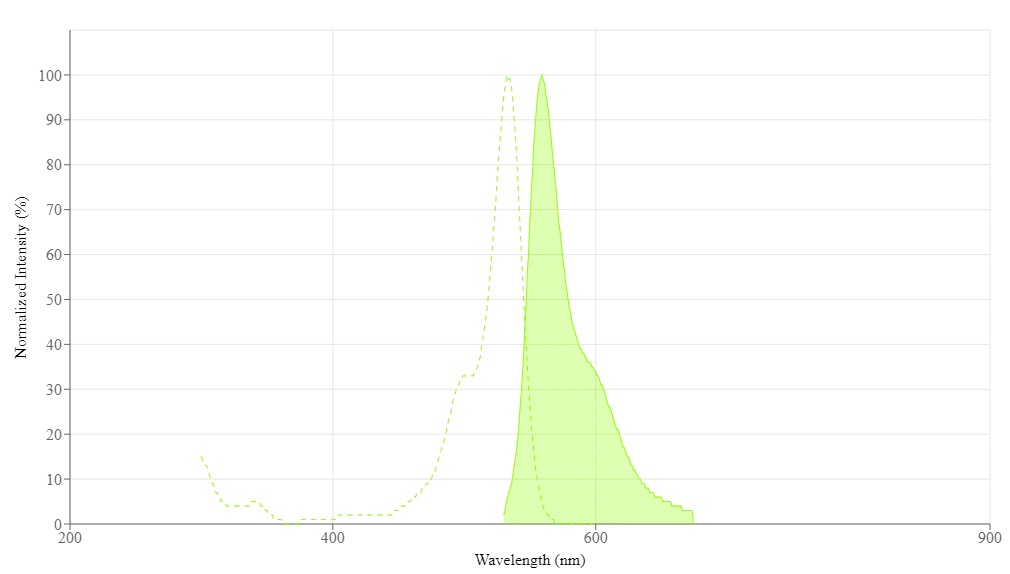
Propiedades Espectrales
| Factor de corrección (280 nm) | 0.15 |
| Coeficiente de extinción (cm -1 M -1) | 73000 |
| Excitación (nm) | 533 |
| Emisión (nm) | 550 |
Imagenes

Figura 1. Estructura quimica de 6-HEX phosphoramidite [5′-Hexachlorofluorescein phosphoramidite]
Productos Relacionados
| Nombre | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Correction Factor (280 nm) |
| 6-HEX, SE [6-Carboxy-2′,4,4′,5′,7,7′-hexachlorofluorescein, succinimidyl ester] *Single Isomer* | 533 | 559 | 73000 | 0.15 |
| 6-HEX azide | 533 | 559 | 73000 | 0.15 |
| 6-HEX alkyne | 533 | 559 | 73000 | 0.15 |
| 6-FAM phosphoramidite [5′-Fluorescein phosphoramidite] *CAS 204697-37-0* | 493 | 517 | 83000 | 0.178 |
| 6-Fluorescein phosphoramidite | 498 | 517 | 800001 | 0.275 |
| 6-TET phosphoramidite [5′-Tetrachlorofluorescein phosphoramidite] *CAS#: 877049-90-6* | 521 | 542 | 73000 | 0.13 |
| 6-JOE Phosphoramidite | 520 | 545 | 750002 | – |
| HEX-dT Phosphoramidite | – | – | – | – |
Referencias
Sequence-specific, self-reporting hairpin inversion probes
Authors: Browne KA., undefined
Journal: J Am Chem Soc (2005): 1989
Modified oligonucleotides containing lithocholic acid in their backbones: their enhanced cellular uptake and their mimicking of hairpin structures
Authors: Kim SJ, Bang EK, Kwon HJ, Shim JS, Kim BH.
Journal: Chembiochem (2004): 1517
Synthesis of HyBeacons and dual-labelled probes containing 2′-fluorescent groups for use in genetic analysis
Authors: Dobson N, McDowell DG, French DJ, Brown LJ, Mellor JM, Brown T.
Journal: Chem Commun (Camb) (2003): 1234
Reduced aggregation and improved specificity of G-rich oligodeoxyribonucleotides containing pyrazolo[3,4-d]pyrimidine guanine bases
Authors: Kutyavin IV, Lokhov SG, Afonina IA, Dempcy R, Gall AA, Gorn VV, Lukhtanov E, Metcalf M, Mills A, Reed MW, S and ers S, Shishkina I, Vermeulen NM.
Journal: Nucleic Acids Res (2002): 4952
Diels–Alder bioconjugation of diene-modified oligonucleotides
Authors: Hill KW, Taunton-Rigby J, Carter JD, Kropp E, Vagle K, Pieken W, McGee DP, Husar GM, Leuck M, Anziano DJ, Sebesta DP.
Journal: J Org Chem (2001): 5352
Recovery of an oligonucleotide using silver ions immobilized onto paramagnetic particles
Authors: Ramirez-Vick JE, Garcia AA, Lee J.
Journal: Prep Biochem Biotechnol (1998): 243
Simultaneous detection of several oligonucleotides by time-resolved fluorometry: the use of a mixture of categorized microparticles in a sandwich type mixed-phase hybridization assay
Authors: Hakala H, Virta P, Salo H, Lonnberg H.
Journal: Nucleic Acids Res (1998): 5581
Synthesis and characterization of a new 5-thiol-protected deoxyuridine phosphoramidite for site-specific modification of DNA
Authors: Meyer KL, Hanna MM.
Journal: Bioconjug Chem (1996): 401
Synthesis and characterisation of fluorescent oligonucleotides. Effect of internal labelling on protein recognition
Authors: Hagmar P, Bailey M, Tong G, Haralambidis J, Sawyer WH, Davidson BE.
Journal: Biochim Biophys Acta (1995): 259
Characterization of endonucleolytic activity of HIV-1 integrase using a fluorogenic substrate
Authors: Lee SP, Censullo ML, Kim HG, Knutson JR, Han MK.
Journal: Anal Biochem (1995): 295
Application Notes (en Ingles)
A Novel Fluorescent Probe for Imaging and Detecting Hydroxyl Radical in Living Cells
Abbreviation of Common Chemical Compounds Related to Peptides
Annexin V
Bright Tide Fluor™-Based Fluorescent Peptides and Their Applications In Drug Discovery and Disease Diagnosis
Calcein


