Descripción
Los isómeros TAMRA individuales son cada vez más preferidos para marcar péptidos y nucleótidos porque brindan una mejor resolución en la purificación por HPLC que a menudo se requiere en los procesos de conjugación. 6-TAMRA, SE se utiliza predominantemente para marcar nucleótidos y ácidos nucleicos.
| Catalogo | Producto | Presentación |
|---|---|---|
| AAT-376 | 6-TAMRA, SE [6-Carboxytetramethylrhodamine, succinimidyl ester] | 5 mg |
| AAT-377 | 6-TAMRA, SE [6-Carboxytetramethylrhodamine, succinimidyl ester] | 100mg |
| AAT-378 | 6-TAMRA, SE [6-Carboxytetramethylrhodamine, succinimidyl ester] | 1 g |
Importante: Solo para uso en investigación (RUO). Almacenamiento: Congelar (< -15 °C); Minimizar la exposición a la luz
Propiedades fisicas
| Peso Molecular | 527.53 |
| Disolvente | DMSO |
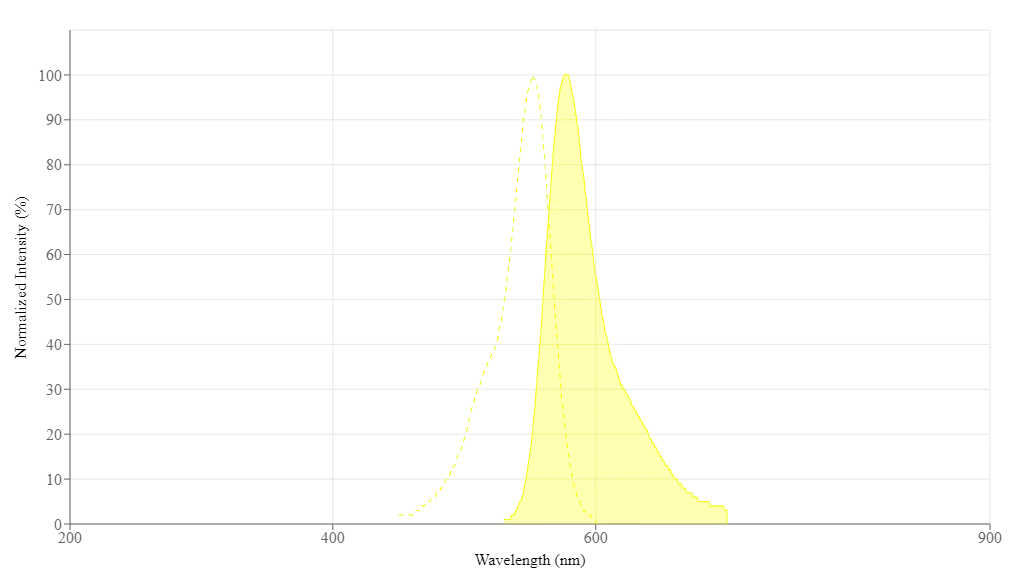
Espectro
Abrir en Advanced Spectrum Viewer
Propiedades espectrales
| Factor de correción (260 nm) | 0.32 |
| Factor de correción (280 nm) | 0.178 |
| Coeficiente de extinción (cm -1 M -1) | 90000 |
| Exitación (nm) | 552 |
| Emisión (nm) | 578 |
Calculadora
Preparación de la solución de stock común
Volumen de DMSO necesario para reconstituir la masa específica de 6-TAMRA, SE [6-carboxitetrametilrodamina, succinimidil éster] *CAS#: 150810-69-8* a la concentración dada. Tenga en cuenta que el volumen es solo para preparar la solución madre. Consulte el protocolo experimental de muestra para conocer los buffers experimentales/fisiológicos apropiados.
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 189.563 µL | 947.813 µL | 1.896 mL | 9.478 mL | 18.956 mL |
| 5 mM | 37.913 µL | 189.563 µL | 379.125 µL | 1.896 mL | 3.791 mL |
| 10 mM | 18.956 µL | 94.781 µL | 189.563 µL | 947.813 µL | 1.896 mL |

Figura 1. Estructura quimica de 6-TAMRA, SE [6-Carboxytetramethylrhodamine, succinimidyl ester] *CAS#: 150810-69-8*
Productos Relacionados
| Nombre | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Correction Factor (260 nm) | Correction Factor (280 nm) |
| 6-CR6G, SE [6-Carboxyrhodamine 6G, succinimidyl ester] *Single isomer* | 522 | 546 | 94000 | 0.24 | 0.214 |
| 5(6)-TAMRA [5(6)-Carboxytetramethylrhodamine] *CAS 98181-63-6* | 552 | 578 | 90000 | 0.32 | 0.178 |
| 5-TAMRA, SE [5-Carboxytetramethylrhodamine, succinimidyl ester] *CAS#: 150810-68-7* | 552 | 578 | 90000 | 0.32 | 0.178 |
| 6-ROX, SE [6-Carboxy-X-rhodamine, succinimidyl ester] *CAS#: 216699-36-4* | 578 | 604 | 82000 | – | 0.168 |
| 6-TAMRA Maleimide [Tetramethylrhodamine-6-maleimide] *CAS 174568-68-4* | 552 | 578 | 90000 | 0.32 | 0.178 |
| 6-FAM, SE [6-Carboxyfluorescein, succinimidyl ester] *CAS 92557-81-8* | 493 | 517 | 83000 | 0.32 | 0.178 |
| 6-HEX, SE [6-Carboxy-2′,4,4′,5′,7,7′-hexachlorofluorescein, succinimidyl ester] *Single Isomer* | 533 | 559 | 73000 | – | 0.15 |
| 6-JOE, SE [6-Carboxy-4′,5′-dichloro-2′,7′-dimethoxyfluorescein, succinimidyl ester] *CAS#: 113394-23-3* | 520 | 545 | 750001 | – | – |
| 6-TET, SE [6-Carboxy-2′,4,7′,7-tetrachlorofluorescein, succinimidyl ester] | 521 | 542 | 73000 | – | 0.13 |
Bibliografía
Thiol-ene conjugation of VEGF peptide to electrospun scaffolds as potential application for angiogenesis
Authors: Yao, Tianyu and Chen, Honglin and Wang, Rong and Rivero, Rebeca and Wang, Fengyu and Kessels, Lilian and Agten, Stijn M and Hackeng, Tilman M and Wolfs, Tim GAM and Fan, Daidi and others,
Journal: Bioactive Materials (2023): 306–317
Design and Characterization of Two Bifunctional Cryptophane A-Based Host Molecules for Xenon Magnetic Resonance Imaging Applications
Authors: Rossella, Federica and Rose, Honor May and Witte, Christopher and Jayapaul, Jabadurai and Schröder, Leif
Journal: ChemPlusChem (2014): 1463–1471
Human erythrocytes as drug carriers: loading efficiency and side effects of hypotonic dialysis, chlorpromazine treatment and fusion with liposomes
Authors: Favretto, ME and Cluitmans, JCA and Bosman, GJCGM and Brock, R
Journal: Journal of Controlled Release (2013): 343–351
Advances in Quantitative FRET-Based Methods for Studying Nucleic Acids
Authors: Preus, Søren and Wilhelmsson, L Marcus
Journal: ChemBioChem (2012): 1990–2001
Referencias
Optical detection of DNA hybridization based on fluorescence quenching of tagged oligonucleotide probes by gold nanoparticles
Authors: Wu ZS, Jiang JH, Fu L, Shen GL, Yu RQ.
Journal: Anal Biochem (2006): 22
Masking oligonucleotides improve sensitivity of mutation detection based on guanine quenching
Authors: Maruyama T, Shinohara T, Hosogi T, Ichinose H, Kamiya N, Goto M.
Journal: Anal Biochem (2006): 8
A fluorescence polarization assay for screening inhibitors against the ribonuclease H activity of HIV-1 reverse transcriptase
Authors: Nakayama GR, Bingham P, Tan D, Maegley KA.
Journal: Anal Biochem (2006): 260
High efficiency micellar electrokinetic chromatography of hydrophobic analytes on poly(dimethylsiloxane) microchips
Authors: Roman GT, McDaniel K, Culbertson CT.
Journal: Analyst (2006): 194
Metal-enhanced fluorescence-based RNA sensing
Authors: Aslan K, Huang J, Wilson GM, Geddes CD.
Journal: J Am Chem Soc (2006): 4206
Fluorescence anisotropy and FRET studies of G-quadruplex formation in presence of different cations
Authors: Juskowiak B, Galezowska E, Zawadzka A, Gluszynska A, Takenaka S.
Journal: Spectrochim Acta A Mol Biomol Spectrosc (2006): 835
Electrostatic-gated transport in chemically modified glass nanopore electrodes
Authors: Wang G, Zhang B, Wayment JR, Harris JM, White HS.
Journal: J Am Chem Soc (2006): 7679
A targeted protease substrate for a quantitative determination of protease activities in the endolysosomal pathway
Authors: Fischer R, Bachle D, Fotin-Mleczek M, Jung G, Kalbacher H, Brock R.
Journal: Chembiochem (2006): 1428
G Quadruplex-Based FRET Probes with the Thrombin-Binding Aptamer (TBA) Sequence Designed for the Efficient Fluorometric Detection of the Potassium Ion
Authors: Nagatoishi S, Nojima T, Galezowska E, Juskowiak B, Takenaka S.
Journal: Chembiochem (2006): 1730
Fluorescence properties of fluorescein, tetramethylrhodamine and Texas Red linked to a DNA aptamer
Authors: Unruh JR, Gokulrangan G, Wilson GS, Johnson CK.
Journal: Photochem Photobiol (2005): 682
Application Notes (en Ingles)
Abbreviation of Common Chemical Compounds Related to Peptides


