Descripción
Luminol [3-aminoftalhidrazida] es un sustrato de peroxidasa luminiscente. Para exhibir su luminiscencia, el luminol debe activarse con un oxidante. HRP cataliza la descomposición del peróxido de hidrógeno.
Cuando el luminol reacciona con el ion hidróxido, se forma un dianión. El oxígeno producido a partir del peróxido de hidrógeno luego reacciona con el dianión de luminol. El producto de esta reacción, un peróxido orgánico, es muy inestable y se produce por la pérdida de nitrógeno, el cambio de electrones del estado excitado al estado fundamental y la emisión de energía en forma de fotón. Esta emisión produce el resplandor azul.
| Catalogo | Producto | Presentación |
|---|---|---|
| AAT-11050 | Luminol [3-Aminophthalhydrazide] *CAS 521-31-3* | 1 gr |
Importante, Solo para uso en investigación (RUO). Almacenamiento: Congelación a <-15 °C. MInimizar la exposición a la luz.
Propiedades Fisicas
| Peso Molecular | 177.16 |
| Disolvente | DMSO |
Calculadora
Preparación de la solución de stock común
Volumen de DMSO necesario para reconstituir la masa específica de Luminol [3-aminoftalhidrazida] CAS 521-31-3 a la concentración dada. Tenga en cuenta que el volumen es solo para preparar la solución madre. Consulte el protocolo experimental de muestra para conocer los buffers experimentales/fisiológicos apropiados.
| 0.1 mg | 0.5 mg | 1 mg | 5 mg | 10 mg | |
| 1 mM | 564.462 µL | 2.822 mL | 5.645 mL | 28.223 mL | 56.446 mL |
| 5 mM | 112.892 µL | 564.462 µL | 1.129 mL | 5.645 mL | 11.289 mL |
| 10 mM | 56.446 µL | 282.231 µL | 564.462 µL | 2.822 mL | 5.645 mL |
Espectro
Abrir en Advanced Spectrum Viewer

Propiedades Espectrales
| Excitación (nm) | 355 |
| Emision (nm) | 412 |
Imagen
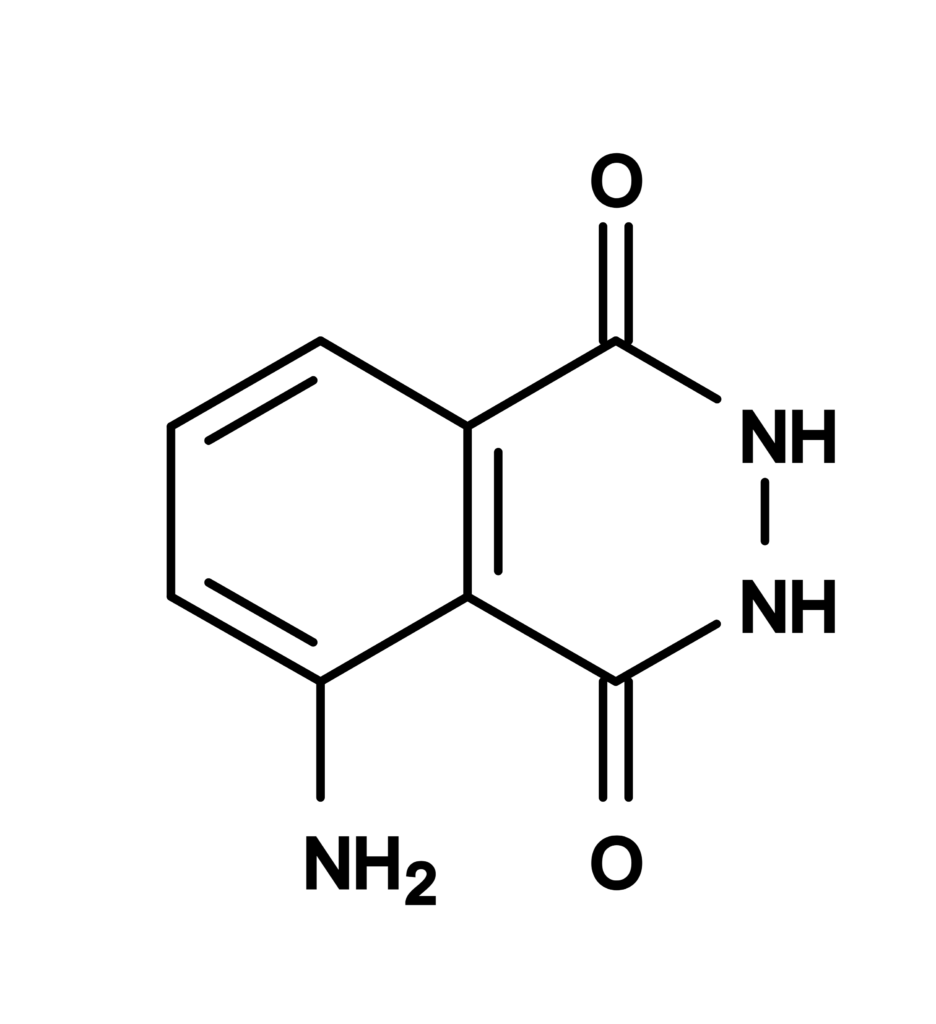
Figura 1. Estructura quimica para Luminol [3-Aminophthalhydrazide] *CAS 521-31-3*
Bibliografía
Linker histone H1. 2 and H1. 4 affect the neutrophil lineage determination
Authors: Sollberger, Gabriel and Streeck, Robert and Apel, Falko and Caffrey, Brian Edward and Skoultchi, Arthur I and Zychlinsky, Arturo
Journal: Elife (2020): e52563
Transaldolase 1 is required for Neutrophil Extracellular Trap (NET) Formation
Authors: Morath, Jakob Paul
Journal: (2020)
Linker Histone H1 subtypes specifically regulate neutrophil differentiation
Authors: Sollberger, Gabriel and Streeck, Robert and Caffrey, Brian Edward and Zychlinsky, Arturo
Journal: bioRxiv (2019): 763482
Gasdermin D plays a vital role in the generation of neutrophil extracellular traps
Authors: Sollberger, Gabriel and Choidas, Axel and Burn, Garth Lawrence and Habenberger, Peter and Di Lucrezia, Raffaella and Kordes, Susanne and Menninger, Sascha and Eickhoff, Jan and Nussbaumer, Peter and Klebl, Bert and others,
Journal: Science immunology (2018): eaar6689
Referencias
Ver todas las 14 referencias: Citation Explorer
Luminol-hydrogen peroxide chemiluminescence produced by sweet potato peroxidase
Authors: Alpeeva IS, Yu Sakharov I.
Journal: Luminescence. (2006)
Ultrasensitive assay of azithromycin in medicine and bio-fluids based on its enhanced luminol-H(2)O(2) chemiluminescence reaction using flow injection technique
Authors: Song Z, Wang C.
Journal: Bioorg Med Chem (2003): 5375
Application of an enhanced luminol chemiluminescence reaction using 4-[4,5-di(2-pyridyl)-1H-imidazol-2-yl]phenylboronic acid to photographic detection of horseradish peroxidase on a membrane
Authors: Kuroda N, Murasaki N, Wada M, Nakashima K.
Journal: Luminescence (2001): 167
Comparative studies of the chemiluminescent horseradish peroxidase-catalysed peroxidation of acridan (GZ-11) and luminol reactions: effect of pH and scavengers of reactive oxygen species on the light intensity of these systems
Authors: Osman AM, Zomer G, Laane C, Hilhorst R.
Journal: Luminescence (2000): 189
New phenylboronic acid derivatives as enhancers of the luminol-H(2)O(2)-horseradish peroxidase chemiluminescence reaction
Authors: Kuroda N, Kawazoe K, Nakano H, Wada M, Nakashima K.
Journal: Luminescence (1999): 361
What do we measure with luminol-, lucigenin- and penicillin-amplified chemiluminescence? 1. Investigations with hydrogen peroxide and sodium hypochlorite
Authors: Rost M, Karge E, Klinger W.
Journal: J Biolumin Chemilumin (1998): 355
Enhancer effect of fluorescein on the luminol-H2O2-horseradish peroxidase chemiluminescence: energy transfer process
Authors: Navas Diaz A, Gonzalez Garcia JA, Lovillo J.
Journal: J Biolumin Chemilumin (1997): 199
Synthesis and characterization of 4-iodophenylboronic acid: a new enhancer for the horseradish peroxidase-catalyzed chemiluminescent oxidation of luminol
Authors: Kricka LJ, Cooper M, Ji X.
Journal: Anal Biochem (1996): 119
Assay of cholinesterase using a pro-enhancer of the luminol-H2O2-horseradish peroxidase reaction
Authors: Navas Diaz A, Garcia Sanchez F, Gonzalez Garcia JA, Bracho Del Rio V.
Journal: J Biolumin Chemilumin (1995): 285
4-Phenylylboronic acid: a new type of enhancer for the horseradish peroxidase catalysed chemiluminescent oxidation of luminol
Authors: Kricka LJ, Ji X.
Journal: J Biolumin Chemilumin (1995): 49
Application notes
A Meta-Analysis of Common Calcium Indicators
A New Protein Crosslinking Method for Labeling and Modifying Antibodies
A Novel Fluorescent Probe for Imaging and Detecting Hydroxyl Radical in Living Cells
Abbreviation of Common Chemical Compounds Related to Peptides
Annexin V
FAQ
What is enhanced chemiluminescence?
Are there any alternatives to BrdU (Bromodeoxyuridine)?
Are there any alternatives to Cy5?
Are there any alternatives to indocyanine green (ICG)?
Can DAPI bind to RNA?
AssayWise
Selecting the right ROS probe
HRP Antibody Labeling Using Buccutite™ Crosslinking Technology
iFluor® 700 Dyes
Hydroxyl Radical Detection
Peroxidase Detection


